The global clinical research ecosystem is undergoing a structural redesign. What began as an emergency response during the COVID-19 pandemic has evolved into a sustained transition toward hybrid and decentralized trial models, approaches that blend physical trial sites with digital, remote, and community-based components.
This shift is no longer marginal. The decentralized and hybrid clinical trials market is projected to more than double globally by the end of the decade, driven by sponsor demand for faster recruitment, improved retention, and operational resilience.
For Africa, however, the relevance of this shift is not about trend adoption. It is about design sovereignty: whether new trial models will be imposed wholesale, or shaped deliberately around local realities, infrastructure, and long-term system building.
What Hybrid Clinical Trials Mean in the African Context
Hybrid clinical trials combine:
- On-site clinical activities: Hospitals, laboratories, investigator-led assessments
- Decentralized elements: Telemedicine, remote monitoring, electronic data capture, and community-based follow-up
In mature markets, hybridization is often driven by convenience and speed. In Africa, however, the drivers are more complex: access, feasibility, trust, and system resilience.

Infrastructure readiness varies significantly between and within countries. While a growing number of African countries now host internationally accredited research sites, only a subset of the continent’s 54 countries consistently conduct registered interventional trials, reflecting uneven capacity distribution rather than lack of demand.
Regulatory maturity differs across jurisdictions. Digital literacy and community trust in research remain uneven. As a result, hybrid trials in Africa cannot be monolithic.
Critical insight:
Hybrid trials in Africa will not be a single operating model. They will emerge as region-specific, capacity-aligned hybrids, anchored in physical research infrastructure and selectively augmented by decentralized tools.
Africa’s Clinical Trial Landscape: Signals Beyond the Headlines
Rather than focusing on aggregate burden statistics, a more useful lens is trajectory.
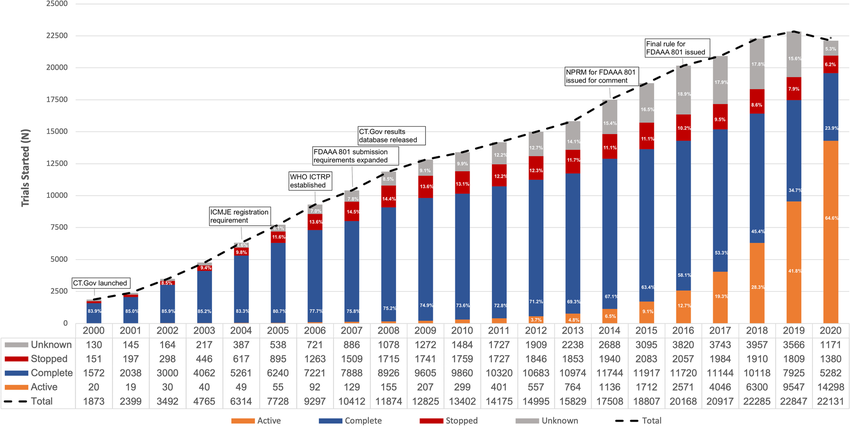
- Over the past two decades, the number of registered clinical trials conducted on the continent has grown from well under 100 per year in the early 2000s to well over 2,000 cumulative registered trials, indicating a steady ecosystem expansion.
- Trial activity remains concentrated in a limited number of countries, with South Africa, Egypt, Kenya, Nigeria, and Uganda consistently emerging as regional anchors for clinical research.
- Regulatory harmonization efforts such as joint ethics review mechanisms and the gradual operationalization of the African Medicines Agency are improving predictability for multi-country studies.
- Sponsors are increasingly piloting hybrid components in infectious disease, vaccine, and post-market surveillance studies, rather than attempting full decentralization.
These signals point not to saturation, but to early-stage system formation.
For investors and policymakers, this phase is often the most consequential: it determines who shapes standards, workflows, and trust relationships.
Why Hybrid Trials Make Strategic Sense for Africa
- Access Without Abandoning Structure
Hybrid models can reduce participant travel burdens and improve retention while preserving investigator oversight and laboratory quality. This is especially relevant in regions where fully decentralized trials would weaken clinical governance.
Evidence from global trial implementations shows that hybrid designs can improve participant retention rates and reduce protocol deviations benefits that are particularly valuable in resource-constrained environments.
- Operational Flexibility for Sponsors
By reducing unnecessary site visits and enabling selective remote follow-ups, hybrid designs can shorten recruitment timelines and ease pressure on overstretched tertiary hospitals without compromising data integrity.
For sponsors operating in Africa, this flexibility is not just a cost consideration, it is a risk mitigation strategy.
- More Representative, Higher-Value Data
Africa’s population diversity is increasingly recognized as a scientific asset. Hybrid trials enable broader inclusion while maintaining protocol rigor, strengthening the global relevance of resulting datasets.
As noted in a public statement by the World Health Organization:
“Well-designed, locally led clinical trials are essential to building resilient health systems and ensuring equitable access to innovation.”
Hybrid models, when responsibly implemented, align with this objective.
The Structural and Ethical Challenges, and the Need for Design-Led Solutions
- Digital Infrastructure Is Uneven
Connectivity, power stability and device access remain inconsistent, particularly outside major urban centers.
Design response:
Hybrid-lite approaches anchored in accredited sites and laboratories, with decentralized components deployed selectively, are more realistic than full virtualization in the near to medium term.
- Regulatory Fragmentation Persists
Multiple ethics committees, data governance regimes, and approval timelines increase complexity, especially for multi-country studies.
Design response:
Early regulatory engagement and locally grounded trial management partners reduce friction and improve predictability, an increasingly important factor for institutional investors evaluating execution risk.
Capacity and Skills Gaps Are Real
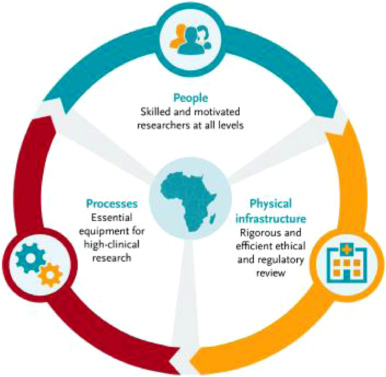
Hybrid trials demand new competencies from:
- Investigators
- Study coordinators
- Data and quality teams
- Participants
Without structured training and operational support, hybridization can increase risk rather than reduce it.
Design response:
This is where organisations like Xcene Research play a critical role.
The Role of Xcene Research in Africa’s Hybrid Trial Future
Xcene Research operates at the intersection of research design, operational execution, and ecosystem collaboration. Rather than treating hybrid trials as a technology problem, the organisation approaches them as a systems challenge.
By working alongside investigators, laboratories, sponsors, and regulators, Xcene Research supports:
- Context-aware hybrid trial design
- Integration of laboratory and diagnostic capabilities
- Capacity building for trial teams
- Alignment between global sponsor expectations and local execution realities
This collaborative model recognizes a simple truth:
Hybrid trials succeed not because they are digital, but because they are designed for the environments in which they operate.
Are Hybrid Trials Already Happening in Africa?
Yes, but selectively.
Hybrid elements are increasingly present in:
- Vaccine trials
- Infectious disease studies
- Pragmatic and post-market research
While Africa still represents a small single-digit percentage of global clinical trial activity, hybrid components are already being tested across multiple regions. What is emerging is not mass adoption, but proof of feasibility.
The next phase will be defined by scale, standardization, and trust.
So, Are Hybrid Trials the Future for Africa?
Hybrid trials are not the future of Africa by default but they may become the future Africa designs for itself.
That future depends on:
- Infrastructure investment that matches ambition
- Regulatory evolution driven by collaboration, not compliance alone
- Local research institutions acting as co-architects, not subcontractors
- Community engagement embedded from trial conception
For governments, this is a policy opportunity.
For sponsors, a strategic frontier.
For investors, a chance to participate early in the construction of Africa’s next-generation clinical research ecosystem.
Partner with the future of clinical research in Africa.
Xcene Research – Your Indigenous CRO partner with local expertise and global impact across sub-Saharan Africa.