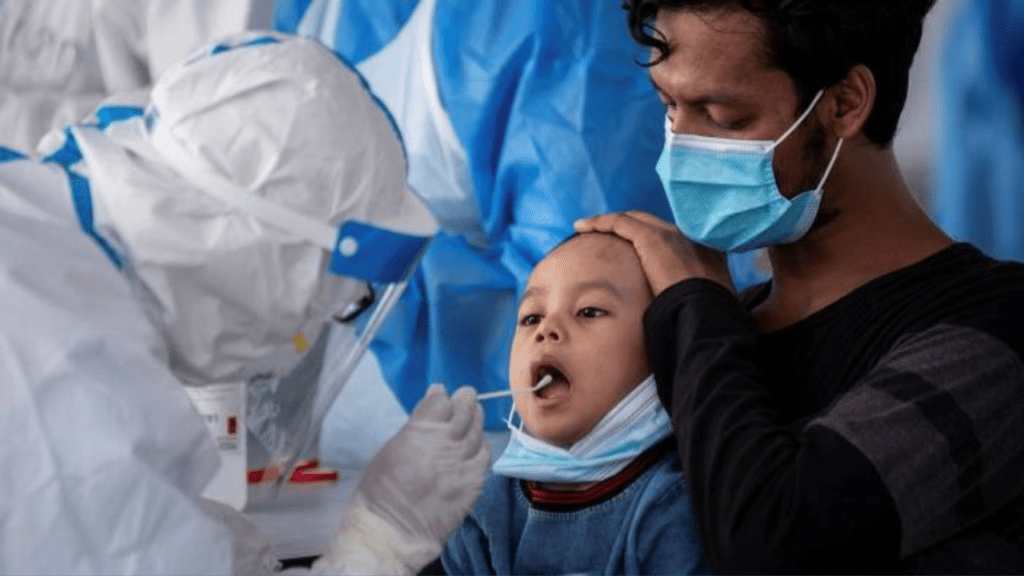
Why aren’t children included in most COVID-19 Trials?
There are several reasons why children are widely excluded from current COVID-19 studies. In general, pediatric studies present more challenges than traditional adult studies. For starters, there are simply fewer patients to recruit. The pediatric population makes up only about 17% of the world population, and pediatric studies must segment age ranges within studies to ensure the safety of the participants.
These studies usually separate participants into four age groups: <2, 2-5, 6-11, and 12-17 years old. Additionally, some conditions do not occur as frequently in children as they do in adults, which further reduces the population size for recruitment. On top of that, some conditions that occur in both children and adults present differently in each, often making it more difficult to identify and diagnose certain conditions in children.
Logistically, pediatric studies require the buy-in and participation of entire families. Studies must get both permission (informed consent) from the patient’s parent or legal guardian, as well as assent from the patient herself. Of course, consent is only part of the challenge. Retaining pediatric patients is difficult, especially when a study requires multiple site visits that children must be driven to or miss school for.
Inevitably, the responsibility often falls on the parent to ensure their child is following study and treatment protocols. This combined with other common family elements—siblings with their own schedules and needs, single-parent households, and demanding work schedules, for example—can make participating in a pediatric trial a logistical challenge for both the patient and the rest of the family.
On top of the already existing hurdles to pediatric research, COVID-19 trials are being conducted during a global pandemic where social distancing is greatly encouraged. This raises even more questions and concerns.
Many parents wonder if it is safe for their child to participate—could they be putting themselves or more vulnerable family members at greater risk by participating? Site visits that once allowed parents to attend with children may need to be rethought, and the use of mobile technology will likely be necessary.
Evidence from the first months of the pandemic suggests that, although children and adults likely are just as susceptible to infection, the risk of severe disease appears to be lower in children. However, serious cases of COVID-19 have been reported in children, including neonates, young infants, and those with underlying medical conditions.
It is also important to note that testing has been much more limited in children, which could be responsible for the relatively low number of confirmed pediatric cases. As testing increases across the globe, so does the rate of positive cases in children.
Initially, priority was placed on providing care for those most severely impacted by the disease, including elderly and immuno-compromised patients. While emphasizing these at-risk populations is merited, we must also start identifying and filling the key knowledge gaps regarding COVID-19 infection in pediatric populations so that we can better address the needs of children.
Including children in COVID-19 research will improve our understanding of the virology, innate, and acquired immune responses in children, helping us to develop therapies that improve outcomes in infected kids. Perhaps most importantly, including children in research may enable us to significantly reduce the spread of infection moving forward.