As global clinical trials expand into more diverse and complex environments, Africa is increasingly part of sponsors’ strategic planning. Yet operational considerations continue to shape how and where studies are ultimately executed.
From regulatory timelines and site readiness to logistics coordination, workforce capacity, and data oversight, the risks sponsors encounter in Africa are real. Importantly, however, they are not unique, nor are they insurmountable. In most cases, they reflect execution gaps rather than structural limitations and they can be mitigated through informed planning and strong local partnerships.
With active operations across Sub-Saharan Africa and headquarters in Nigeria, Xcene Research works at the intersection of global trial expectations and local operational realities. Below, we examine the most common operational risks sponsors face in Africa and the mitigation strategies that consistently work in practice.
1. Fragmented Regulatory and Ethical Processes
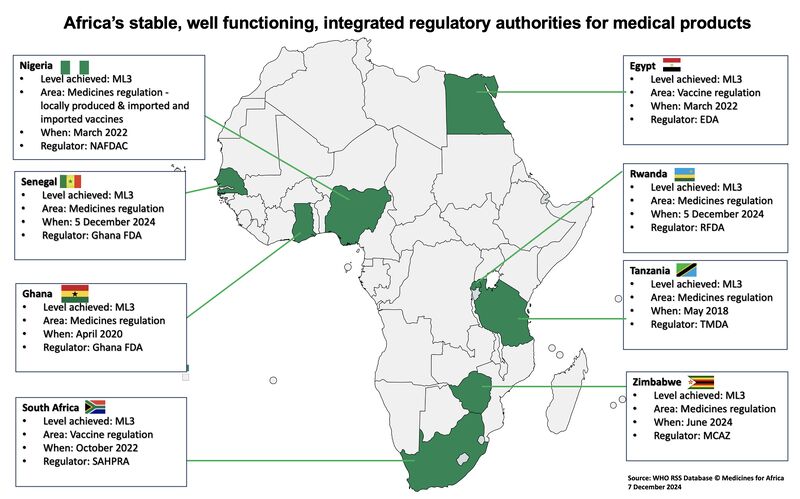
Clinical trials in Africa are regulated at the national level, with each country operating its own regulatory authority and ethics review framework. Unlike regions with centralized systems, sponsors must navigate multiple approval pathways, often involving national regulators, institutional ethics committees, and in some cases regional or ministerial oversight.
Recent regulatory assessments show that approval timelines across African countries can vary from as little as 60 days to well over a year, depending on submission quality, therapeutic area, and regulator capacity. This variability is often perceived as regulatory inefficiency, but in reality, it reflects jurisdictional diversity and differing maturity levels across systems.
In practice, delays most often arise when regulatory strategy is developed too late or without country-specific insight.
Mitigation That Works
- Regulatory mapping during feasibility: Identify each country’s submission requirements, review cycles and timelines at the outset.
- Dedicated regulatory intelligence: Partner with advisors or in-region specialists who have established relationships with national authorities.
- Tailored submissions: Where possible, develop a harmonized dossier with country-specific addenda to reduce back-and-forth during review.
Effective mitigation hinges on early regulatory intelligence, realistic timeline modeling, and partnerships with teams that understand how approvals actually move within each country.
2. Supply Chain and Logistics Complexity
Africa’s infrastructure landscape is highly heterogeneous. While some urban centers support advanced cold-chain logistics, others particularly in semi-rural or emerging research regions present challenges related to transport reliability, customs clearance, and temperature control.
Clinical supply chain analyses indicate that logistics-related issues account for up to 30% of trial delays in emerging markets, frequently due to underestimated customs requirements or insufficient cold-chain planning. These risks are compounded when import permits, tax waivers, and customs documentation are initiated too close to first shipment.
Mitigation That Works
- Local logistics partners: Engage couriers with deep operational knowledge of customs procedures and clinical supply requirements in each market.
- Cold chain verification: Validate cold chain infrastructure across transit points and sites before initiating shipment.
- Pre-clearance planning: Start import permits and customs documentation early to align with expected delivery windows.
3. Site Readiness and Infrastructure Variability
Africa’s clinical research capacity is growing, but it remains unevenly distributed. While countries such as South Africa, Kenya, and Nigeria host well-established research centers, assessments of laboratory readiness across Sub-Saharan Africa show that fewer than 400 laboratories meet internationally recognized quality standards, with over 90% concentrated in South Africa.
Sponsors relying solely on checklist-based feasibility assessments risk overestimating readiness particularly around power stability, internet reliability, laboratory accreditation, and workflow maturity.
Mitigation That Works
- Beyond checklist feasibility: Assess power reliability, connectivity, laboratory accreditation status, and workflow readiness not just facility presence.
- Capacity strengthening: Fund or coordinate training, equipment upgrades, and digital system deployments aligned with GCP (Good Clinical Practice).
- Phased activation: Implement staged site initiation with increased oversight during early enrolment to ensure quality before full rollout.
With focused investment and phased activation, capable sites can be brought to global standards while expanding sponsor access to diverse patient populations.
4. Human Resource Capacity and Training Gaps
Clinical research depends heavily on people investigators, coordinators, monitors, and data personnel. Across many African markets, demand for trained research professionals is growing faster than formal training pipelines, and staff turnover can affect continuity if not actively managed.

Globally, regulatory inspections continue to identify protocol deviations and documentation gaps as leading causes of audit findings issues that are closely tied to training and experience rather than geography.
Xcene Research addresses this risk by integrating role-specific GCP and protocol training into study startup and maintaining refresher programs throughout trial conduct. In several West African studies, pairing less experienced coordinators with senior mentors reduced query rates and improved protocol adherence within the first enrollment cohorts.
The operational insight is clear: investing in people early reduces downstream quality risk far more effectively than retrospective monitoring.
5. Community Engagement and Recruitment Risk
Large population size does not guarantee rapid recruitment. In many African settings, recruitment challenges arise from limited trial awareness, socio-cultural considerations, and historical mistrust, particularly where communities have had limited prior exposure to research.
Evidence from community-based research initiatives shows that trials without structured engagement strategies are significantly more likely to experience enrollment delays exceeding 20%–30% of planned timelines.
Mitigation That Works
- Context-aligned engagement: Work with local leaders, healthcare professionals, and grassroots advocates to design recruitment approaches that resonate culturally.
- Trial literacy campaigns: Educate communities on trial purpose, rights, and benefits to foster informed participation.
- Real-time monitoring: Track enrolment data and adapt outreach tactics quickly when initial strategies plateau.
6. Data Quality and Safety Oversight
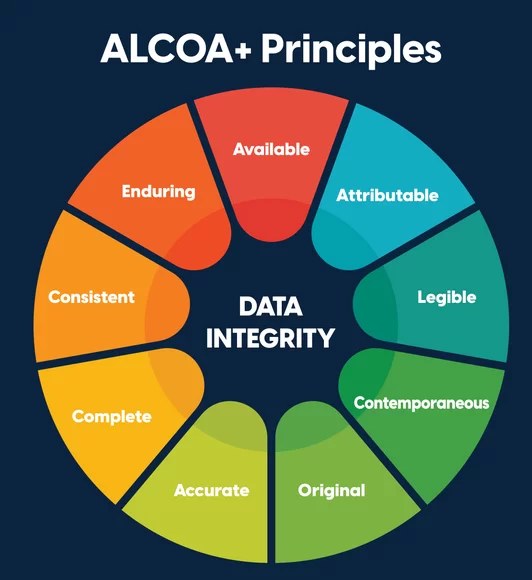
Concerns about data credibility remain one of the most persistent misconceptions surrounding African trials. Yet regulators consistently emphasize that data quality is determined by systems, training, and oversight not location.
The increasing adoption of risk-based monitoring (RBM) and centralized data review has demonstrated measurable benefits, including fewer critical findings and improved inspection readiness across global trials.
Mitigation That Works
- Digital data capture: Use electronic data capture systems aligned with global standards.
- Risk-based monitoring: Blend centralized data review with targeted on-site visits for key risk indicators.
- Safety reporting systems: Implement robust digital safety reporting tools and clear escalation mechanisms.
Evidence of Impact
Sponsors adopting risk-based monitoring report fewer critical queries and stronger inspection outcomes versus conventional plans focused solely on frequent visits.
Conclusion: Operational Risk Is Manageable with the Right Execution Model
Operational risk in Africa is often framed as inherent. In reality, it is contextual and manageable.
Sponsors that approach African trials with informed planning, realistic assumptions, and experienced local execution partners are achieving predictable timelines, high-quality data, and successful regulatory outcomes.
At Xcene Research, we help sponsors move from perception to operational clarity by:
- Providing real-time operational visibility across African trial sites
- Navigating country-specific regulatory and logistics complexities
- Building site capacity and human capital
- Driving data quality and safety monitoring excellence
Africa’s potential in clinical research is vast. Realizing it depends not on avoiding risk but on managing it with insight, experience, and local expertise.
To learn how Xcene Research can support your next study with operational excellence across Sub-Saharan Africa, contact our team. info@xceneresearch.com