The landscape of clinical trials in Sub-Saharan Africa (SSA) in 2026 is poised for transformative growth. Despite historically representing only a fraction of global research, less than 3% of worldwide trials, the region is emerging as a pivotal frontier in healthcare innovation. With a population exceeding 1.1 billion and bearing 25% of the global disease burden, SSA offers unparalleled opportunities for diverse, inclusive research that addresses local health challenges while contributing to global advancements. This article explores the key trends, breakthroughs, challenges, and predictions shaping clinical trials in SSA this year, drawing on recent data and initiatives to envision a more equitable future in global health. As a leading indigenous Contract Research Organization (CRO), Xcene Research is at the forefront of this evolution, driving innovation and capacity building across the continent.
Accelerating Market Growth and Investment
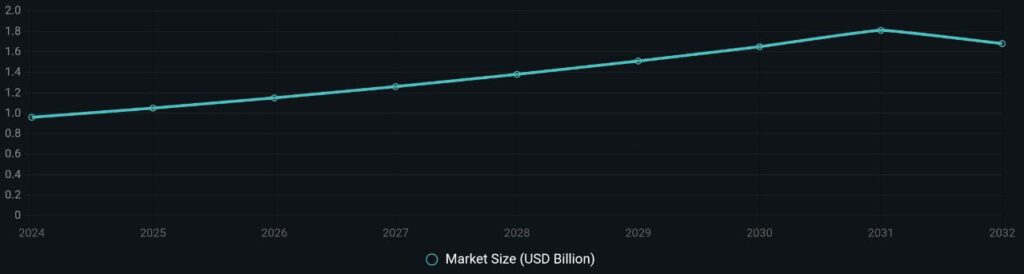
The clinical trials market in Africa is on an upward trajectory, projected to expand from USD 0.96 billion in 2024 to USD 1.68 billion by 2032, reflecting a compound annual growth rate (CAGR) that underscores increasing investor confidence. In SSA specifically, this growth is driven by cost-competitive operations, unsaturated patient pools, and a shift in foreign direct investment (FDI) toward pharmaceutical projects. Recent analyses show that SSA’s share of African pharma FDI has dominated since the early 2010s, with developing economies now leading investments over traditional developed ones.
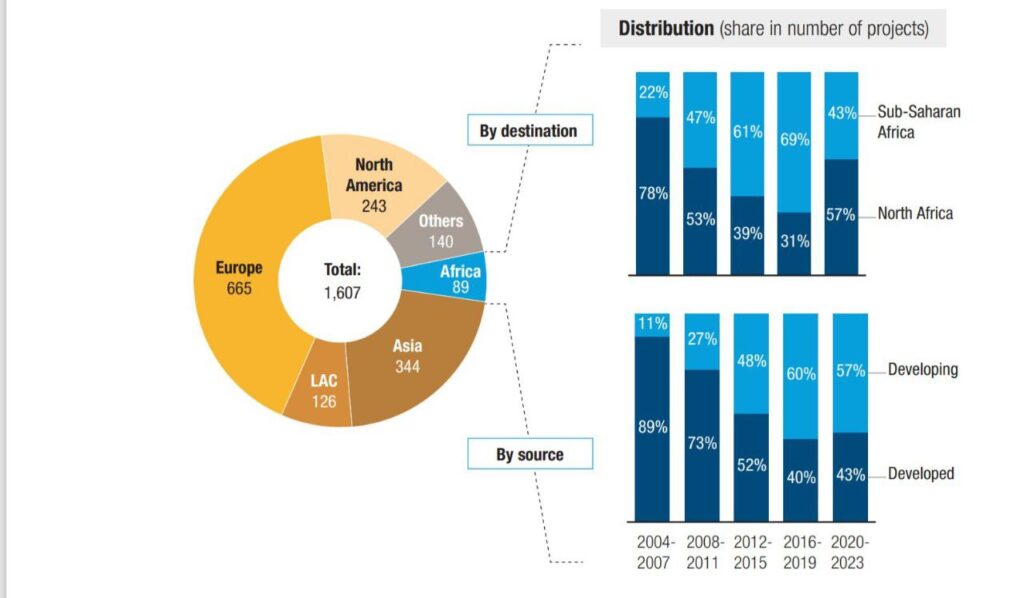
Source: UNCTAD Secretariat, based on information from the Financial Times Ltd, fDi Markets (www.fDmarkets.com)
This expansion is not just economic; it’s strategic. Sponsors and contract research organizations (CROs) like Xcene Research are increasingly turning to SSA to meet diversity mandates in clinical research, ensuring therapies are effective across genetically varied populations. For 2026, expect a surge in trials for neglected tropical diseases, non-communicable diseases (NCDs) like diabetes and hypertension, and infectious threats such as mpox and malaria. Initiatives like the African Medicines Agency (AMA) will further streamline regulatory processes, reducing timelines and attracting more global partnerships.
Xcene Research, with its focus on indigenous expertise and end-to-end services, is advocating for expanded trials in SSA, making Africa a compelling destination for cutting-edge research.
To illustrate this momentum, consider the projected market growth:
Embracing Technological Innovations
2026 will mark a pivotal year for technology integration in SSA clinical trials. Artificial intelligence (AI) and machine learning (ML) are set to revolutionize trial design, patient recruitment, and real-time data analytics. Predictions indicate widespread adoption of eClinical technologies, including decentralized trials that leverage mobile health (mHealth) tools to overcome geographical barriers in remote areas.
For instance, AI-powered platforms will optimize site selection and predictive modeling, reducing dropout rates that currently average 43% in chronic disease trials. In SSA, where infrastructure challenges persist, these innovations will enable hybrid models combining virtual monitoring with on-site visits. Partnerships with organizations like the European & Developing Countries Clinical Trials Partnership (EDCTP) and local CROs, such as Xcene Research, will amplify this by building digital-ready sites and providing GCP training and supporting standardization efforts essential for equity and global credibility.
Moreover, the efforts to integrate blockchain for data integrity and AI-driven pharmacovigilance Will get stronger, addressing ethical concerns, and ensuring transparency in a region historically plagued by exploitation in trials.
Prioritizing Diversity, Equity, and Local Leadership
A core theme for 2026 is inclusivity. Global regulators, including the FDA and EMA, are pushing for diverse trial populations to combat biases in drug development. SSA’s genetic diversity makes it indispensable for this, yet only 1.1% of global trials occurred here in 2023. This year, anticipate a rise in Africa-led trials, focusing on pandemic preparedness and NCDs.
Local capacity building is key. Programs emphasizing staff development, such as those in XRSAN, will help retain talent and reduce the “brain drain.” However, challenges like the enormous gap to match pathologist ratios in high-income countries highlight the need for accelerated training. Thought leaders must advocate for ethical frameworks that prioritize community engagement and informed consent, learning from past controversies.
Spotlight on Breakthroughs and Case Studies
2026 builds on recent successes. In HIV research, trials at the University of KwaZulu-Natal in South Africa have shown 20% of participants achieving viral suppression without ART for over 18 months, signaling progress toward a cure. Similarly, the rollout of the first malaria treatment for infants, approved in 2025, exemplifies how SSA trials can lead to life-saving innovations.
Ethnopharmacology is another bright spot, with African universities translating traditional knowledge into Phase II/III trials for respiratory and infectious diseases. Vaccine trials against mpox, led by CEPI and PATH, will expand in East/Central Africa, enhancing regional preparedness. Xcene Research, as a pioneer in SSA, is expanding its impact by supporting these efforts with local expertise, bridging divides for global health solutions.
Navigating Persistent Challenges
Despite optimism, hurdles remain. Logistics, including cold-chain transport for investigational products, continue to impede progress, especially in remote areas. Regulatory fragmentation and funding gaps, exacerbated by cultural resistance to digital health could slow adoption. Moreover, ethical risks demand vigilant oversight to prevent exploitation.
Addressing these requires higher commitment from governments, pharma, and international bodies. Harmonized regulations via the AMA and increased R&D funding could unlock SSA’s potential, closing the “10/90 gap” where only 10% of research targets 90% of the global disease burden.
Conclusion
In 2026, SSA stands at the cusp of becoming a global leader in clinical trials, driven by innovation, diversity, and collaboration. By leveraging networks like XRSAN and embracing AI, the region can not only participate in but shape the future of healthcare. Xcene Research’s transformative work, encompassing site networks and capacity building, is not only catalyzing and increase in trial volumes but also ensuring sustainable, Africa-centric impacts. Hence, stakeholders must prioritize ethical, inclusive research to ensure benefits accrue locally, fostering healthier communities and economic growth.
As thought leaders, we must champion equitable research that benefits local communities, fostering economic growth and healthier futures. The time for Africa is now.